We use theoretical and computational methods rooted in the physical sciences to investigate the fascinating areas of cell biology, immunology, soft biological materials, and cell-free systems. Our general research interests include:
Intracellular transport
Stochastic and spatial effects in biochemical reaction networks
Cell-free systems
Systems biology
Statistical mechanics
Computational cell biology and immunology
Theory and simulation of soft biological materials
Membrane and polymer biophysics
Antigen recognition by immune cells
We collaborate widely with experimental groups to understand biological structures and processes. Some of our ongoing projects are described in more detail below.
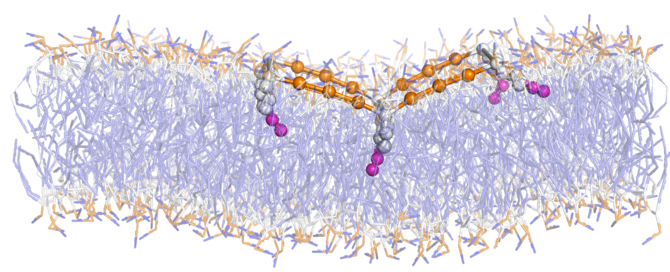
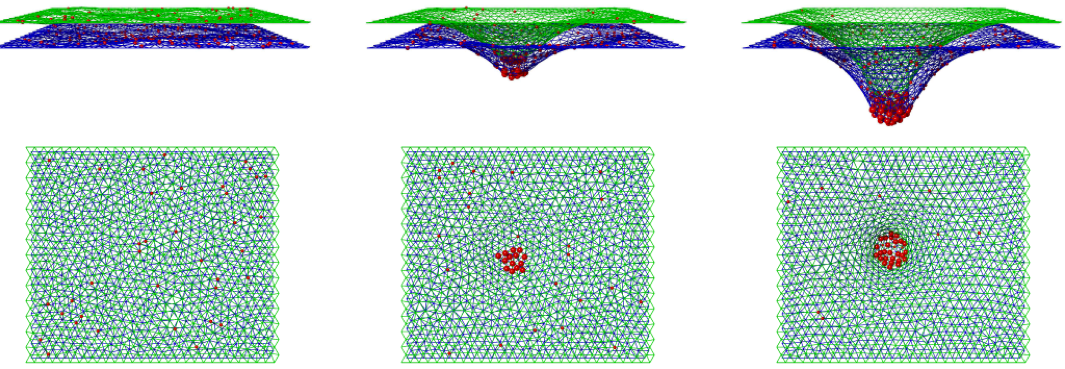
deformable DNA origami nanoparticles on biomembranes
Biomembranes are a valuable platform for organizing nano- and microparticles for applications in biology and soft materials. Recent advances in DNA origami technology have enabled the creation of deformable nanoparticles with precisely controllable shapes and mechanical properties. While the importance of particle flexibility in self-assembly is known in other fields, it has been unexplored in the context of nanoparticle-membrane interactions.
Our objectives are to quantify the energetics of adsorption of deformable DNA origami particles onto lipid membranes and to establish a mechanistic link between particle flexibility, membrane deformability, and the resulting configurations and self-assembly of the particles. We aim to reveal new design principles for the coupled behaviors of biomembranes and deformable, lipid-anchored nanostructures, thus enabling predictive design of nanostructures that respond to, assemble on, and modulate biomembranes in controllable manners.
Support: NSF Award CBET-2217777
Modeling the physical regulation of immune cell activation
One of the central problems in immunology involves molecular recognition at cell-cell interfaces. An emerging paradigm is that mechanical forces regulate processes by which T cells and B cells identify molecular signatures of pathogens. However, theoretical and computational approaches have lagged behind experiments in this rapidly growing field of immunology and are needed to understand collective processes at the cell surface that regulate immune cell activation.
We use theory and computation to investigate how mechanical forces and active processes at the cell surface modulate, and potentially enhance, the ability of immune cells to discriminate between self and foreign ligands on the surfaces of other cells. We focus on how collective dynamics of surface receptors – which control the activation of T cells and B cells – are influenced by physical processes at the cell membrane. We are developing computational models that capture important biophysical interactions at cell-cell interfaces, including stochastic receptor-ligand binding kinetics, membrane mechanics, and actin-mediated forces on the membrane.
Support: NSF CAREER Award PHY-1753017
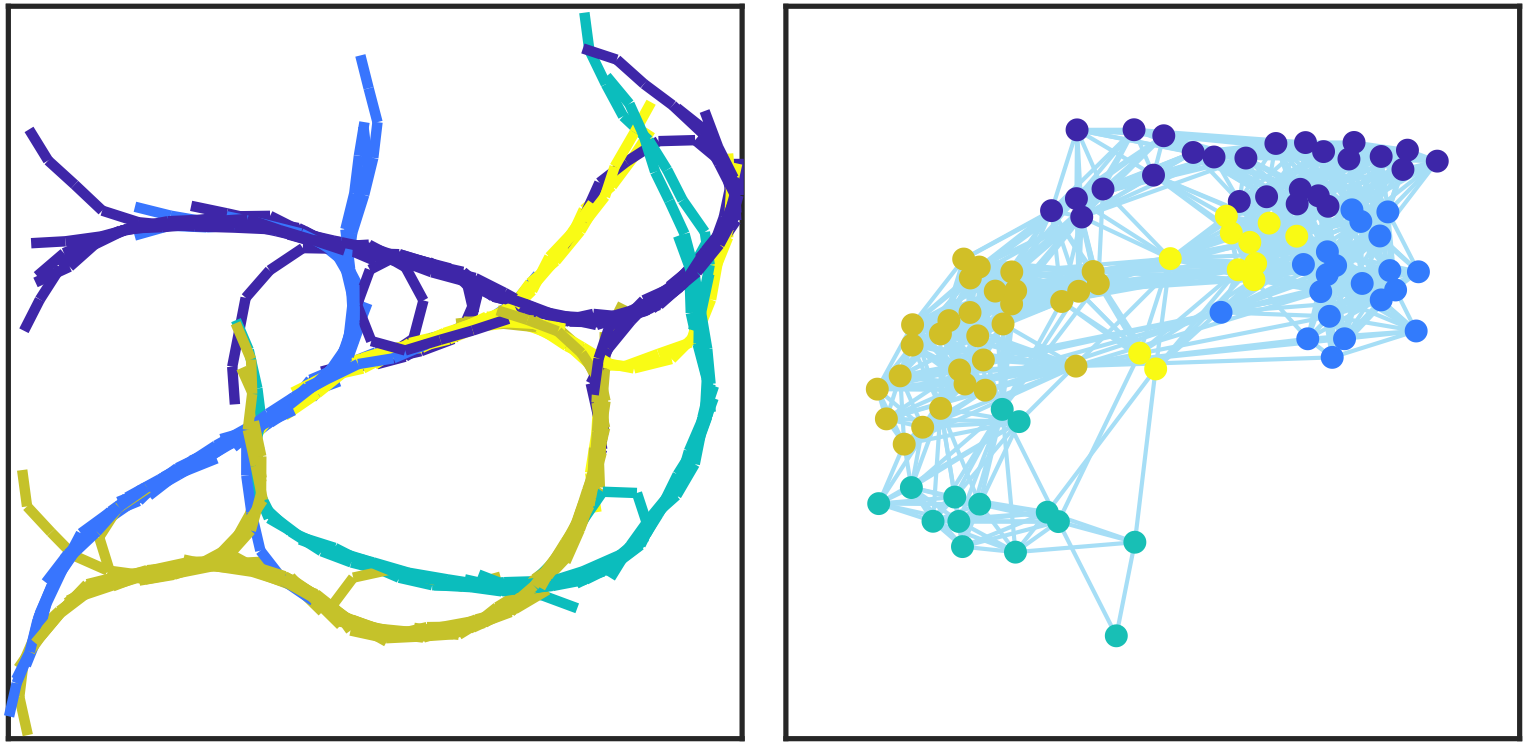
Cytoskeletal organization and intracellular transport
Diffusive processes are too slow for transporting many molecular components within eukaryotic cells, and active transport mechanisms are essential for proper cell function. One striking example in plant cells is cytoplasmic streaming, which is characterized by the rapid movement of organelles along the actin cytoskeleton. While it is known that these movements are driven by myosin motor proteins, the biophysical mechanisms are still debated.
We investigate biophysical mechanisms responsible for organelle transport in cells. We are developing coarse-grained physical models describing (1) the organization and dynamics of the actin cytoskeleton and (2) the dynamics of molecular motors that actively transport cargo along cytoskeletal filaments. We are collaborating with experimentalists to model, measure, and evaluate intracellular dynamics and to discriminate between different possible mechanisms underlying rapid organelle motion and intracellular transport.
Support: NSF Awards MCB-1715794 and MCB-2334517
membrane biophysics and Cell division
Proper cell division is critical for growth, development, and survival of an organism. Defects in cell division have been linked to developmental anomalies and various afflictions. We study cell division in two organisms - fission yeast and bacteria of the genus Chlamydia - to help to illuminate mechanisms by which cells organize their protein machinery, modulate membrane properties, and generate forces to physically separate into daughter cells.
We utilize a variety of methods in our studies of cell division: coarse-grained molecular dynamics simulations to characterize the role of specific phospholipids in shaping local membrane properties, continuum modeling of membranes to characterize their effects on a cellular scale, and spatiotemporal kinetic models to understand organization of proteins that orchestrate cell division. Our studies are conducted in collaboration with experimentalists in biology departments and medical schools.
Support: NSF Awards MCB-1616495 and MCB-1817653, XSEDE and the Pittsburgh Supercomputing Center
Spatial organization and stochastic chemical kinetics in crowded and confined cell-free systems
Cells are highly confined, and much of their volume is occupied by proteins and other macromolecules. Macromolecular crowding and physical confinement have consequences including altered diffusion, modified spatiotemporal correlations between proteins, and large-scale spatial organization of cellular components. These physical effects can dramatically impact cellular processes such as gene expression and other biochemical reaction networks. We are collaborating with experimentalists at Oak Ridge National Laboratory to investigate how crowding and confinement affect both the spatial organization of cellular components and the stochastic dynamics of biochemical reaction networks in cell-free systems.
Support: DOE-ORNL-UT Battelle